41 does sugar ionize in water
Liquid Sugar: Calories, Weight Gain, Blood Sugar, and More - Healthline Here are the calorie and sugar contents in 12 ounces (355 mL) of some popular high sugar beverages: Soda: 151 calories and 39 grams of sugar ( 2. Trusted Source. ) Sweetened iced tea: 144 calories ... Does sugar ionize in water? - AnswersAll Sugar will readily dissolve in water but doesn't form cations and anions in solution. That is, there are no charge carriers formed. Substances that only partially ionize into ions when dissolved in water are called weak electrolytes. For example, sugar dissolves completely in water but it is a non-electrolyte. Does sucrose dissociate in water?
Does sugar ionize or dissociate in water? - urhelpmate.com Glucose (sugar) readily dissolves in water, but because it does not dissociate into ions in solution, it is considered a nonelectrolyte; solutions containing glucose do not, therefore, conduct electricity. Does sugar dissolves easily in water?

Does sugar ionize in water
Solubility and Complex-Ion Equilibria - Purdue University Sugar dissolves in water because energy is given off when the slightly polar sucrose molecules form intermolecular bonds with the polar water molecules. The weak bonds that form between the solute and the solvent compensate for the energy needed to disrupt the structure of both the pure solute and the solvent. 14.2: Ionization of Water - Chemistry LibreTexts 14.2: Ionization of Water. In the section on amphiprotic species, we saw that water can act as a very weak acid and a very weak base, donating protons to itself to a limited extent: The equilibrium constant K for this reaction can be written as follows: where a is the activity of a species. Because water is the solvent, and the solution is ... Can a sugar solution conduct electric current? | Socratic This is an exceedingly small amount of ionization. The water conducts so little current that it is often called a nonconductor. If tap water is used to make the sugar solution, the ions in the tap water will increase the conductivity about 500-fold. It is the water in the sugar solution that has the ability to conduct electricity, not the sugar.
Does sugar ionize in water. Ionic & Covalent Solutes: Definition & Difference | Study.com A covalent solute like sugar does not dissociate into ions at all when it dissolves in water, so it is a non-electrolyte. It will not increase the electrical conductivity of water. It will not ... Dissolution of sugar in water and its temperature dependence You can first consider thermodynamics. Sugar crystals or granules put into water are the initial state and the sugar molecules dissolved in water forming a homogeneous solution are the final state. Since sugar can be dissolved in water, it must be true that the final state is more stable than the initial state, even at elevated temperature. Solvent properties of water (article) | Khan Academy A solvent is simply a substance that can dissolve other molecules and compounds, which are known as solutes. A homogeneous mixture of solvent and solute is called a solution, and much of life's chemistry takes place in aqueous solutions, or solutions with water as the solvent. Because of its polarity and ability to form hydrogen bonds, water ... Water and Aqueous Solutions - Brigham Young University-Idaho However, when polar covalent molecules dissolve in water, they do not ionize or separate into smaller particles like ionic compounds do. Sucrose or table sugar (C 12 H 22 O 11 ) is a good example of a polar compound that readily dissolves in water, forming an aqueous solution.
Chemistry 1050 Chapter 7 Flashcards | Quizlet Add more sugar You have an unsaturated sugar-water solution. To get more sugar to dissolve, you could decreases As temperature of a solution increases, the solubility of a gas in the solution Students also viewed 19 terms 31 terms 5 terms 125 terms Sets found in the same folder 30 terms katelin003 Chemistry 1050 30 terms kelsiejo_smith Does Glucose Form Ions In Water | DiabetesTalk.Net Solubility And Complex-ion Equilibria. The sugar we use to sweeten coffee or tea is a molecular solid, in which theindividual molecules are held together by relatively weak intermolecular forces. Whensugar dissolves in water, the weak bonds between the individual sucrose molecules arebroken, and these C12H22O11 molecules are released ... 7.5: Aqueous Solutions - Chemistry LibreTexts When water dissolves sugar, it separates the individual sugar molecules by disrupting the attractive forces, ... Some other polar molecular compounds become electrolytes upon being dissolved into water but do not ionize to a very great extent. For example, nitrous acid \(\left( \ce{HNO_2} \right)\) only partially ionizes into hydrogen ions and ... Myths and Truth about Water Ionizers - RKIN It was determined by the important data collected that alkaline water which is made by water ionizer was not toxic and can relieve a lot of adult diseases through sugar pill effect. How does water ionizer work? The water ionizer has a fairly straightforward process; these are the steps on how it works:
14.4 Hydrolysis of Salts - Chemistry 2e | OpenStax The Ionization of Hydrated Metal Ions. Unlike the group 1 and 2 metal ions of the preceding examples (Na +, Ca 2+, etc.), some metal ions function as acids in aqueous solutions.These ions are not just loosely solvated by water molecules when dissolved, instead they are covalently bonded to a fixed number of water molecules to yield a complex ion (see chapter on coordination chemistry). Electrolytes | Chemistry for Majors - Lumen Learning Dissolution of an ionic compound is facilitated by ion-dipole attractions between the ions of the compound and the polar water molecules. Soluble ionic substances and strong acids ionize completely and are strong electrolytes, while weak acids and bases ionize to only a small extent and are weak electrolytes. If a sugar is nonreducing, does that mean it doesn't ionize in water? When it does so, it is oxidised. But that doesn't mean that it ionizes in water! When you keep a reducing sugar in the presence of an oxidising agent, the electron is transferred over to the agent, reducing it. The sugar may form an ion, or it may form a carboxylic acid (depending on the medium). Can sugar ionize in water? - Answers No, sugar does not conduct electricity because it can not ionize, so it does not carry a charge. (in the matter of dissolving it in water!) Do acids ionize in water? Strong acids...
Self-ionization of water - Wikipedia The self-ionization of water (also autoionization of water, and autodissociation of water) is an ionization reaction in pure water or in an aqueous solution, in which a water molecule, H 2 O, deprotonates (loses the nucleus of one of its hydrogen atoms) to become a hydroxide ion, OH −.The hydrogen nucleus, H +, immediately protonates another water molecule to form a hydronium cation, H 3 O +.
Ions in Water, and Conductivity - HORIBA Some of you may wonder whether sugar water can also be measured. Unfortunately, a conductivity meter cannot tell you the density of sugar in water. Although sugar is soluble in water, it does not form ions, which means that it is not an electrolyte. ... (CH 3 COONa) is ionized, it becomes the ions acetate CH 3 COO- and sodium ion Na +, but ...
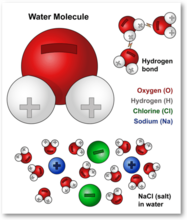
Water molecules and their interaction with salt | U.S ... - USGS The bonds in salt compounds are called ionic because they both have an electrical charge—the chloride ion is negatively charged and the sodium ion is positively charged. Likewise, a water molecule is ionic in nature, but the bond is called covalent, with two hydrogen atoms both situating themselves with their positive charge on one side of ...
Can a sugar solution conduct electric current? | Socratic This is an exceedingly small amount of ionization. The water conducts so little current that it is often called a nonconductor. If tap water is used to make the sugar solution, the ions in the tap water will increase the conductivity about 500-fold. It is the water in the sugar solution that has the ability to conduct electricity, not the sugar.
14.2: Ionization of Water - Chemistry LibreTexts 14.2: Ionization of Water. In the section on amphiprotic species, we saw that water can act as a very weak acid and a very weak base, donating protons to itself to a limited extent: The equilibrium constant K for this reaction can be written as follows: where a is the activity of a species. Because water is the solvent, and the solution is ...
Solubility and Complex-Ion Equilibria - Purdue University Sugar dissolves in water because energy is given off when the slightly polar sucrose molecules form intermolecular bonds with the polar water molecules. The weak bonds that form between the solute and the solvent compensate for the energy needed to disrupt the structure of both the pure solute and the solvent.
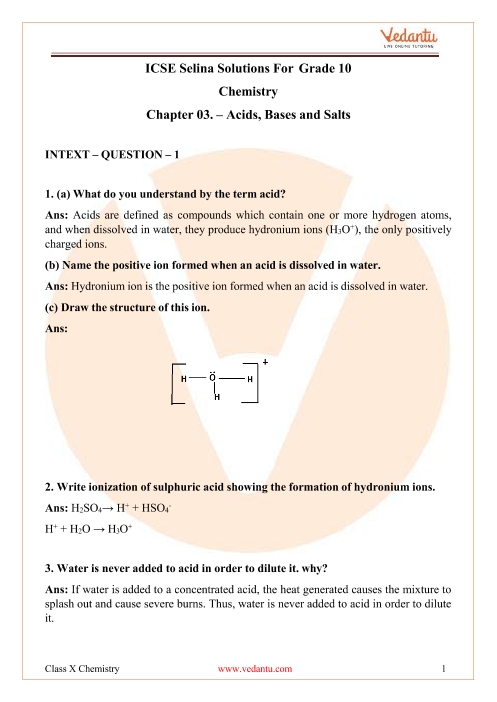



![Calculate the boiling point of solution when 4g of MgSO4 (M = 120 g mol^-1) was dissolved in 100g of water, assuming MgSO4 undergoes complete ionization.[ Kb for water = 0.52 K kg mol^-1]](https://i.ytimg.com/vi/kK4tXHTmVr8/maxresdefault.jpg)

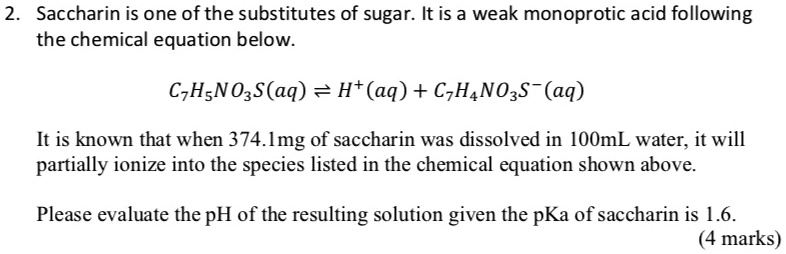


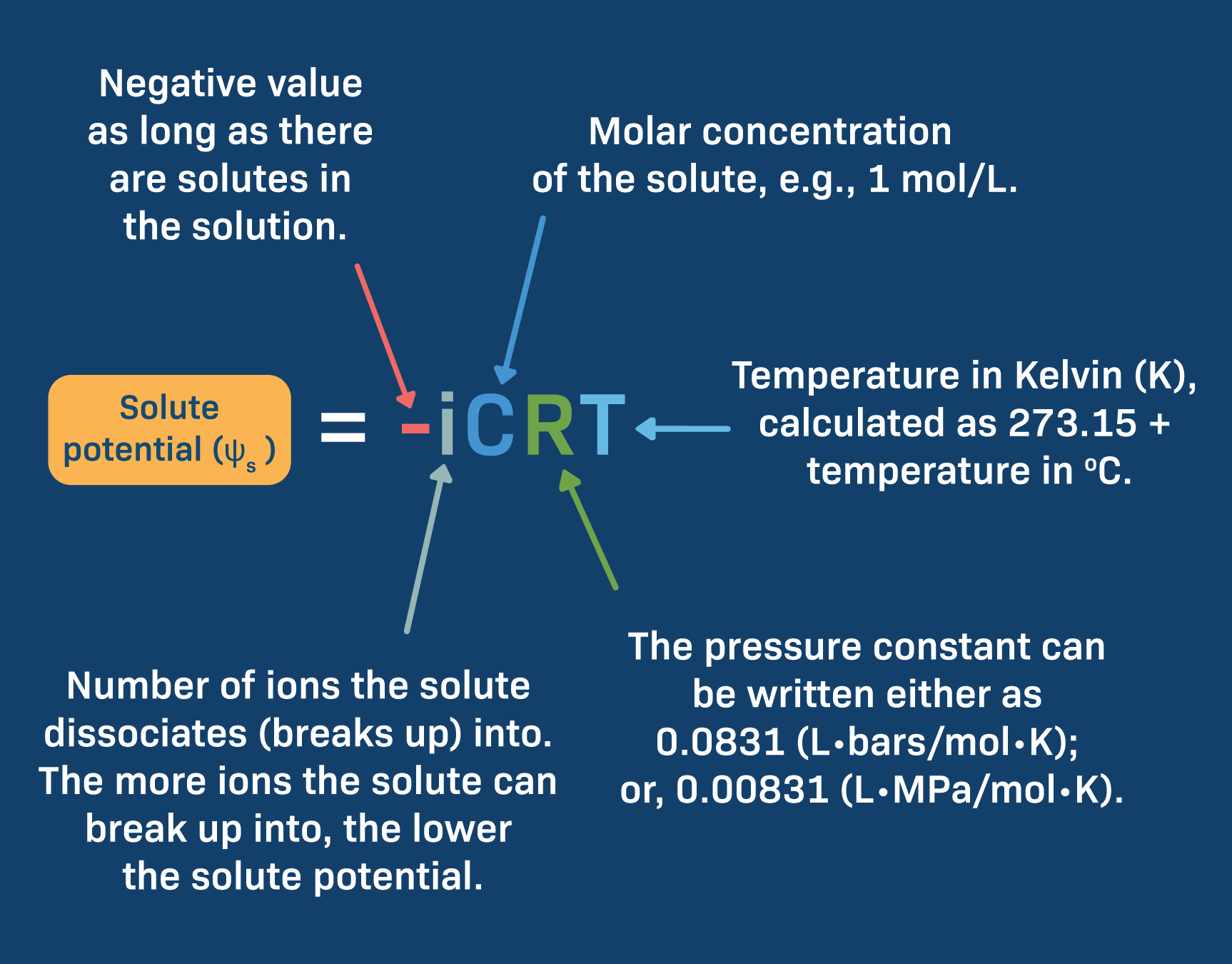





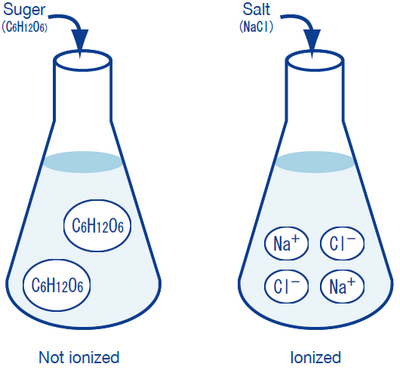
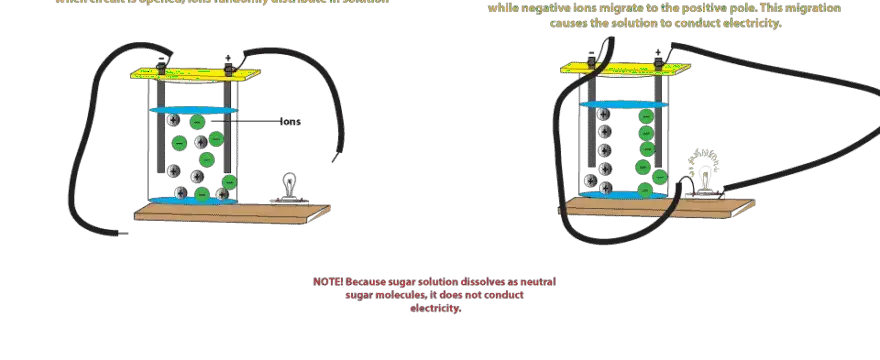










0 Response to "41 does sugar ionize in water"
Post a Comment