42 hot and cold packs chemistry
Cold Pack Chemistry: How Do Cold Packs Work? | Science project ... You can make a basic cold pack by mixing a salt (such as potassium chloride) or soda (such as baking soda) with water. Mixing the two creates a chemical reaction that uses up energy, which makes the mixture colder. You can see which substance cools off water the most by trying out this experiment. Materials: Tap water Measuring cup Cup Amazon.com: Hot And Cold Packs Small Hot Cold 6 Packs, Reusable Round Gel Beads Ice Pack with Cloth Backing, Hot and Cold Therapy for Breastfeed Injury, Kids Pain Relief, Headache, Tired Eyes, Wisdom Teeth, Sinus Relief. 6 Count (Pack of 1) 4.5 out of 5 stars 1,138. $14.99 $ 14. 99 ($14.99/Count) $22.99 $22.99.
PDF Unit 6: Hot and Cold Application Fundamental of Nursing 7. During exercise in hot environment - cool down with fans, ice packs and cold towels Examples of cold applications Cold applications should not be used for longer than 30 minutes at a time. 1. Cold Gel Pack/Ice pack: wrap in a towel to prevent frost bite, place and hold over the area, ice packs contain crushed or chipped ice and are

Hot and cold packs chemistry
Hot and Cold Paks - Chemistry LibreTexts 1. Plastic baggie Metal twist tie 25 g iron metal mesh 100 1 g salt 1 tablespoon small vermiculite 5 mL water Ziploc plastic baggie 20 g sodium thiosulfate (fills one 50 mL beaker) 125 mL warm tap water 250 mL beaker Regular warm ice pack Directions Place iron powder in baggie, add salt and mix contents by shaking (hold baggie closed). Effervescence in chemical reactions - Rates of reaction - BBC Effervescence is an indicator of a chemical reaction taking place. Watch this video to see how magnesium and dilute hydrochloric acid react to produce bubbles of hydrogen gas. Hot and Cold ( Real World ) | Chemistry | CK-12 Foundation Hot and Cold ( Real World ) | Chemistry | CK-12 Foundation Endothermic Reaction Chemical reactions that require energy input to occur. All Modalities Add to Library Share with Classes Details Resources Quick Tips Notes/Highlights Vocabulary Hot and Cold Loading... Found a content error? Tell us Notes/Highlights Image Attributions Show Details
Hot and cold packs chemistry. Hot and Cold Packs in First Aid - Walmart.com Gel Cold & Hot Pack - 14" X 6" Reusable Warm or Ice Pack for Injuries, Hip, Shoulder, Knee, Back Pain - Hot & Cold Compress for Swelling, Bruises, Surgery. 6. Save with. 2-day shipping. $11.29. LotFancy Reusable Gel Ice Pack Wrap with Elastic Strap for Hot Cold Therapy. 2-day shipping. Chemistry in Pictures: Hot and cold chemistry - Chemical & Engineering News Chemistry in Pictures: Hot and cold chemistry. by Craig Bettenhausen. October 3, 2018. Credit: Alexander Lyapunov. Alexander Lyapunov wanted a different perspective on a simple acetone ... Demonstrating The Science Of A Cold Pack Place the empty test tubes in the rack on the scale and zero out the weight. Using the scissors, cut off the top of the cold pack very carefully. Inside the cold pack, you will find a liquid package and white crystals of urea. Remove the liquid package and set it aside. Use the spoon to scoop some of the crystals into a test tube until it reads ... hot sex tube - Search - Bing Web Welcome to Hot Sex TV – the ultimate hot sex tube on the web! Get ready to feast your eyes on scores of naked women having sex right before your eyes. Stream and download movies in high-definition quality. Choose from a wide array of porn videos categories including some of the wildest and kinkiest niches known to man.
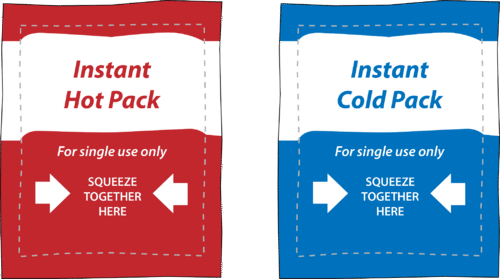
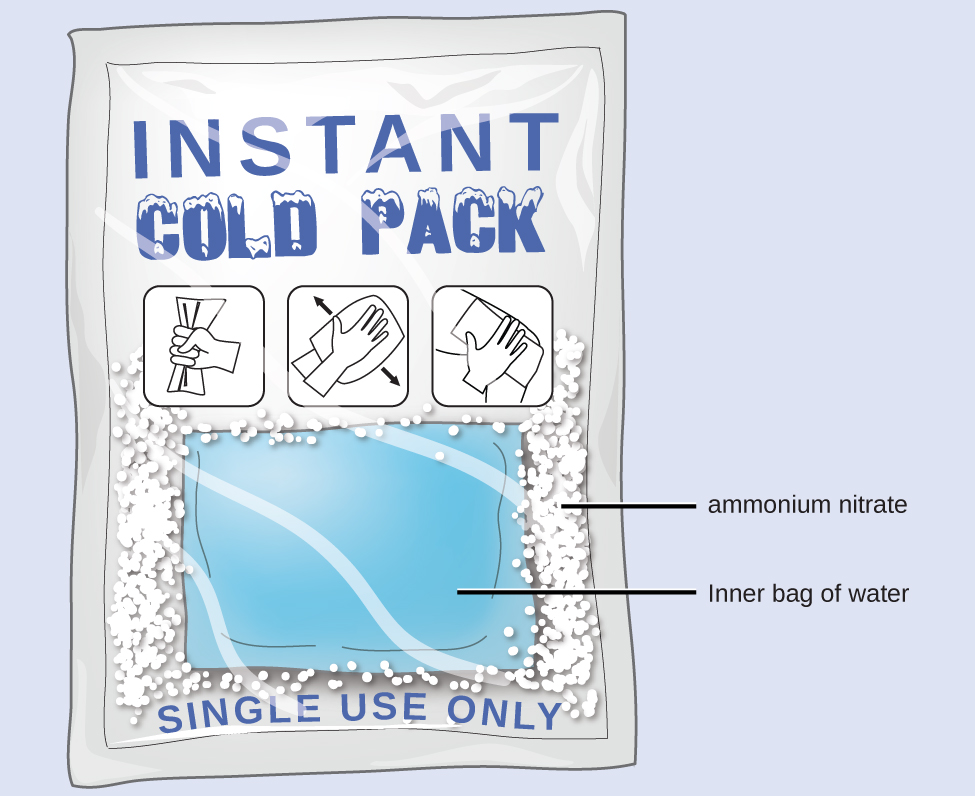
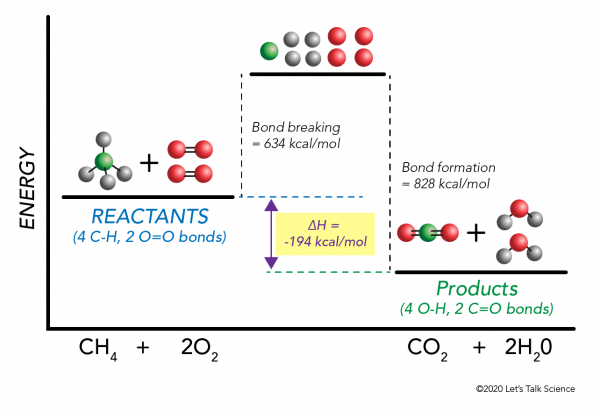
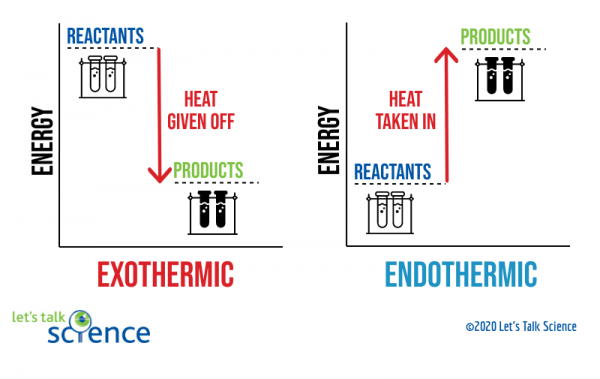
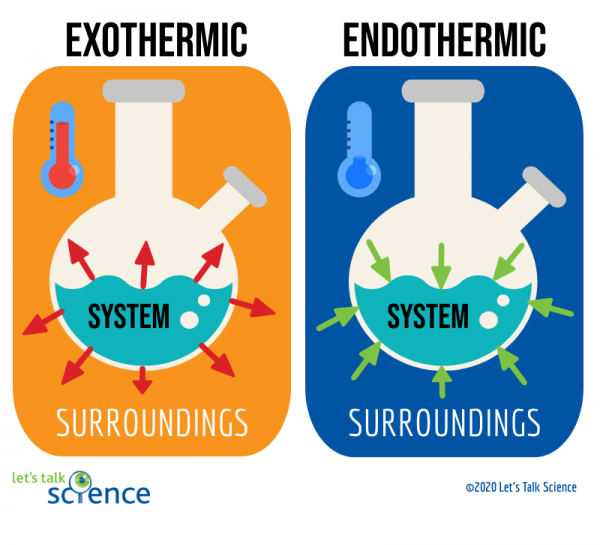
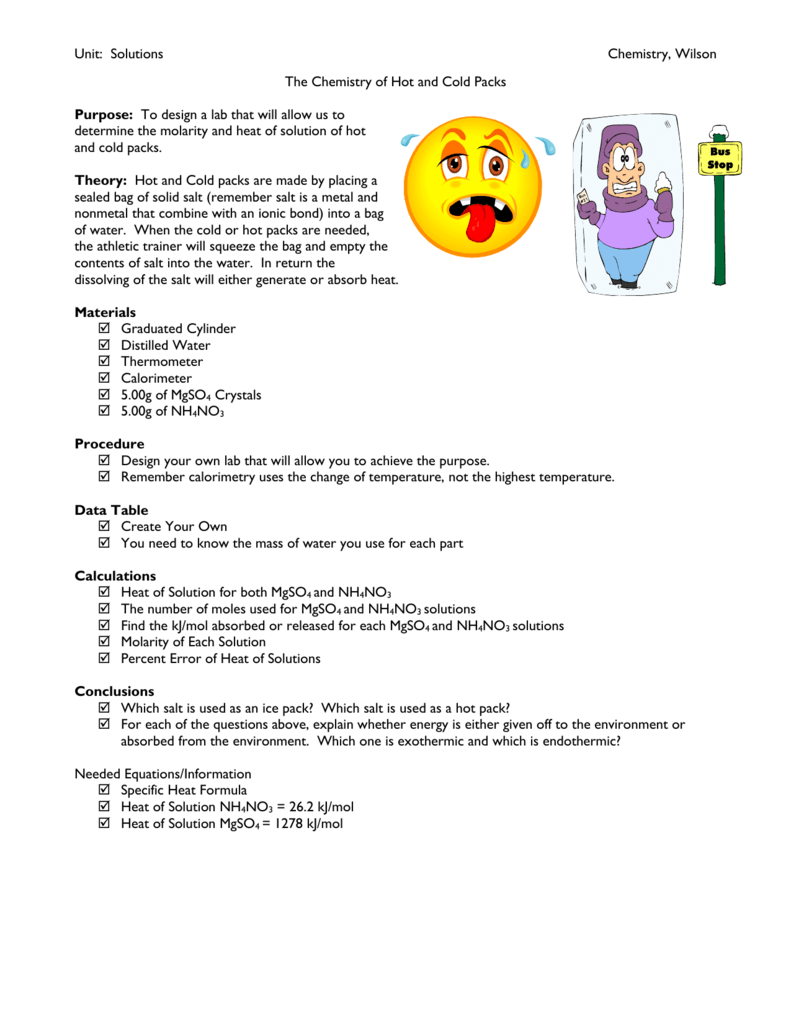
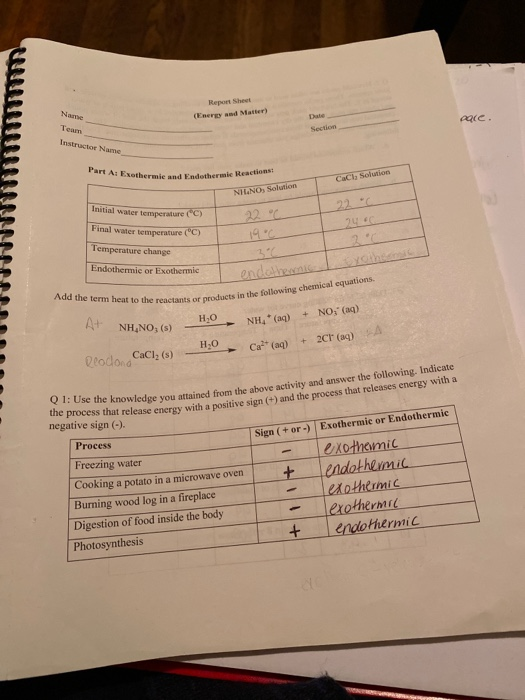
HOT | English meaning - Cambridge Dictionary hot adjective (VERY WARM) A1 having a high temperature: a hot, sunny day hot weather a hot drink / meal It's too hot in here, can we turn down the heating? Bake the cake in a hot oven, about 220°C, for 30 minutes. The food was piping hot (= very hot). Thesaurus: synonyms, antonyms, and examples having a temperature higher than what is comfortable Exothermic and Endothermic Reactions: Hot and Cold Packs Exothermic and Endothermic Reactions: Hot and Cold Packs A small amount of water is added to zip-loc baggies containing either calcium chloride dihydrate or ammonium chloride. The resultant solution becomes hot or cold respectively. The bags are passed around the room so that students can directly sense the temperature changes. Curriculum Notes What Chemicals Are in Hot & Cold Packs? | Healthfully Hot and cold packs contain mixtures of chemicals that react together to create heat and cold 1. ... In single use cold packs, the chemical ammonium nitrate is used. Ammonium nitrate is toxic if ingested. It is a very dangerous chemical used in fertilizer and explosives. If a pack breaks open and the ammonium nitrate comes into contact with ... Hot pack or cold pack: which one to reach for when you're injured or in ... Hot pack or cold pack: which one to reach for when you're injured or in pain Shutterstock Hot pack or cold pack: which one to reach for when you're injured or in pain Published:...
PDF The Chemistry of Heat Packs - University of South Florida heat pack. Evaluate the properties of different plastics (Day 3) Heat seal and test the plastics of their choice. Make the pack. Test the properties of the pack. (Day 4) Introduction / Motivation (5E - Engage) Opening text to students: "Ever been cold on your way to school? Even in Florida, winter mornings can be cold. The chemistry of cold packs - John Pollard | TED-Ed If you stick water in the freezer, it will take a few hours to freeze into ice. How is it, then, that cold packs go from room temperature to near freezing in mere seconds? John Pollard details the chemistry of the cold pack, shedding light on the concepts of energetics and entropy along the way. Watch Think Dig Deeper Discuss The heat is on: heating food and drinks with chemical energy Discussion. Other reactions commonly used in heater meals include the oxidisation of iron, the reaction of anhydrous calcium chloride with water (see below) or, for cooling, the reaction of ammonium nitrate fertiliser with water.. Further experiments such as making your own heat and cold packs, or determining the oxygen content in the air with the aid of the iron oxidation reaction used in ... Developing a New, Improved Cold Pack | Science project - Education Certain chemicals when dissolved in water give off heat, while others become cold. These chemicals can be used in hot or cold packs. Reactions that absorb heat from the environment are called endothermic reactions. These chemicals can be used in hot or cold packs. Cold packs can be used to reduce swelling from a bruise or injury.
HOT | definition in the Cambridge English Dictionary hot adjective (VERY WARM) A1 having a high temperature: a hot, sunny day hot weather a hot drink / meal It's too hot in here, can we turn down the heat? Bake the cake in a hot oven, about 425°F, for 30 minutes. The food was piping hot (= very hot). Thesaurus: synonyms, antonyms, and examples feeling hot hot Are you too hot? I can turn on the air.
Hot and Cold Packs | Fisher Scientific Hot and Cold Packs Devices that retain heat or cold to provide portable temperature modification. Products can be used for applications including body temperature regulation, pain relief, and storage and transport of temperature sensitive specimens. Filter By Hot and Cold Packs 1 - 30 of 67 results 1 3M™ Nexcare™ Reusable ColdHot Gel-Filled Pack 2
Hot Girls in Bikinis - Etsy Hot girl Summer svg, Sunny days svg, Hello Summer svg, Summer time beach svg, Woman bikini svg, Sun svg, Summer svg, Nude svg ad vertisement by BMPvector. Ad vertisement from shop BMPvector. BMPvector From shop BMPvector. 4.5 out of 5 stars (1,219) Sale Price $1.57 $ 1.57 $ 2.24 Original Price $2.24 ...
How Are Hot and Cold Packs Made? - Promotional Products Blog Here's how an ice pack is made: Step One: The refrigerant chemicals are blended together in an industrial mixing machine and left to sit for the materials to fully bind together. Step Two: The empty packs are stamped with usage instructions as well as expiration dates. A logo may be added at this point as well.
Hot and Cold Packs Chem Lab by - Prezi New experiment used to determine the best chemical for use in a hot or cold pack Chemicals tested: Ammonium chloride, calcium chloride, sodium chloride, magnesium sulfate, sodium bicarbonate Our company used a calorimeter to test which of the five chemicals would increase in temperature the most, and which would decrease the most.
Reusable Hot and Cold Gel Ice Packs for Injuries | Cold Compress, Ice ... DUAL PURPOSE HOT AND COLD PACKS - Reusable Ice pack are really handy in the home, can be used for injuries or to keep your lunchbox cool. EXTREMELY DURABLE - Thick medical grade plastic lining and strong seal prevents leaks or punctures. NATURAL & SAFE - The soft ice or heat packs for quick & easy relief. Flexible.
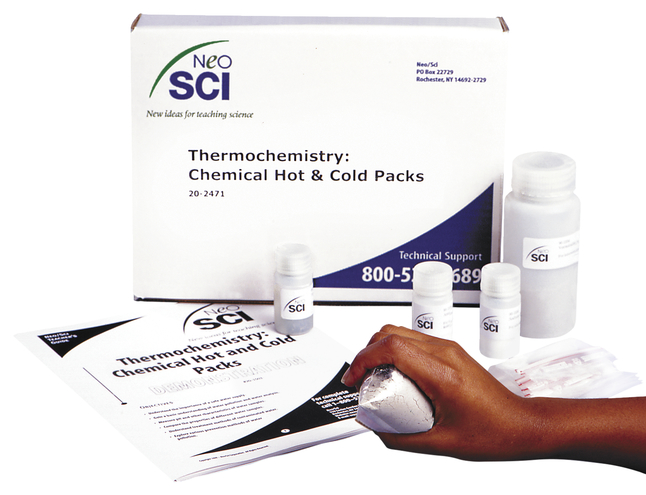
Classroom Resources | Energy in Hot and Cold Packs | AACT The hot packs and cold packs can contain a variety of substances. Choose one that has a packet of water and a solid chemical that is activated by squeezing the pack to break the water pouch so that it mixes with the water. Teaching Points Hot Pack: When the hot pack is activated the dissolving process produces heat, making it an exothermic process.
#hot | TikTok hot | 93.3B views. Watch the latest videos about #hot on TikTok.
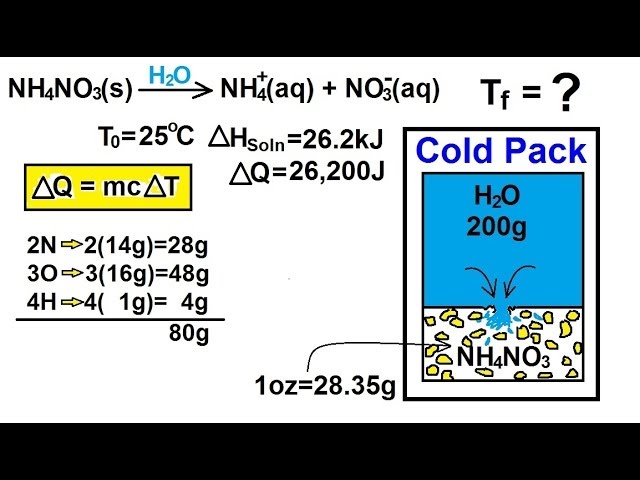
How Does An Instant Cold Pack Work? | Science Project Collect the ammonium nitrate from the instant cold pack, as follows: Put on your safety goggles and latex gloves. Shake the instant cold pack gently to move the water bag and crystals to the bottom. Cut the top of the bag off. Pour the ammonium nitrate crystals into the plastic bowl. Dispose of the water bag.
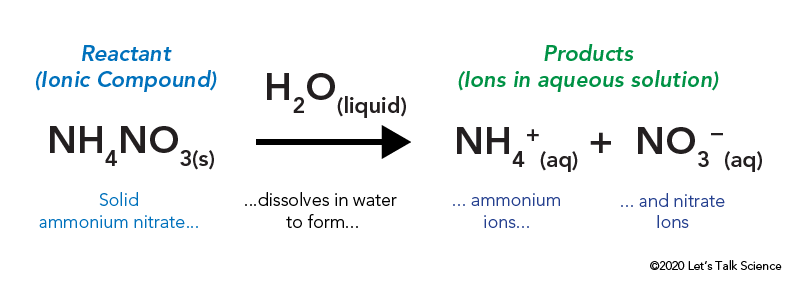
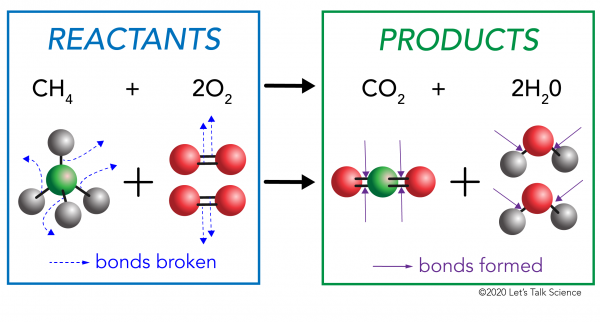
Hot and Cold Packs: A Thermochemistry Activity | Carolina.com Commercial instant cold packs typically use either ammonium nitrate or urea as their salt component; hot packs often use magnesium sulfate or calcium chloride. These reactions happen in a similar manner. When the salt is dissolved in water, the ionic bonds of the salt separate. This process requires energy, which is obtained from the surroundings.
What's Inside an Ice Pack? - Poison Reusable ice packs typically contain water, something to lower the freezing temperature, a thickening agent, silica gel, and non-toxic blue coloring. The concerning component in reusable ice packs is the ingredient used to lower the temperature, which is usually propylene glycol.
All Demos | Chemdemos Heating water from room temperature to boiling water and measuring the temperature of the liquid versus time produces a portion of the Heating Curve for water. Topics: Demonstration Intermolecular Forces Physical Properties States of Matter Read moreabout Boiling Water Demonstration: Heating Curve
DOCX Dirty Business - American Chemical Society This article describes construction and chemistry of the hot and cold packs used for sports injuries. It includes a description of the hydration of salts, including nice diagrams showing water molecules attracting both the positive and the negative ions of the salt. (Marsella, G. Hot & Cold Packs. ChemMatters, 1987, 5 (1), pp 7-11)
Exothermic and Endothermic Reactions: Hot and Cold Packs Exothermic and Endothermic Reactions: Hot and Cold Packs Category: Demonstration Topics: States of Matter Thermodynamics A small amount of water is added to zip-loc baggies containing either calcium chloride dihydrate or ammonium chloride. The resultant solution becomes hot or cold respectively.
12,039,561 Hot Images, Stock Photos & Vectors | Shutterstock Hot royalty-free images 12,033,776 hot stock photos, vectors, and illustrations are available royalty-free. See hot stock video clips Image type Orientation Color People Artists More Sort by Popular Cooking Appliances Physics Space and Astronomy Water Chili pepper Thermometer Air conditioning Steam Temperature of 120,338
Hot and Cold Packs - Lecture Demonstration Manual General Chemistry Commercial heat packs (containing iron and water, or supersaturated sodium acetate) and cold packs (various ammonium salts) can be used to show exo- and endothermicity. Heat packs that contain iron and water packets: Exposing the solution to air results in the oxidation of the iron (creates rust). The oxidation of the iron is an exothermic process.
1,531,305 Hot Women Images, Stock Photos & Vectors - Shutterstock Hot Women royalty-free images 1,529,541 hot women stock photos, vectors, and illustrations are available royalty-free. See hot women stock video clips Image type Orientation Color People Artists More Sort by Popular Clothing and Accessories Women Hair and Skin Diseases, Viruses, and Disorders Woman Lingerie Swimsuit Blond hair Heat stroke Bikini
Hot and Cold ( Real World ) | Chemistry | CK-12 Foundation Hot and Cold ( Real World ) | Chemistry | CK-12 Foundation Endothermic Reaction Chemical reactions that require energy input to occur. All Modalities Add to Library Share with Classes Details Resources Quick Tips Notes/Highlights Vocabulary Hot and Cold Loading... Found a content error? Tell us Notes/Highlights Image Attributions Show Details
Effervescence in chemical reactions - Rates of reaction - BBC Effervescence is an indicator of a chemical reaction taking place. Watch this video to see how magnesium and dilute hydrochloric acid react to produce bubbles of hydrogen gas.
Hot and Cold Paks - Chemistry LibreTexts 1. Plastic baggie Metal twist tie 25 g iron metal mesh 100 1 g salt 1 tablespoon small vermiculite 5 mL water Ziploc plastic baggie 20 g sodium thiosulfate (fills one 50 mL beaker) 125 mL warm tap water 250 mL beaker Regular warm ice pack Directions Place iron powder in baggie, add salt and mix contents by shaking (hold baggie closed).


:max_bytes(150000):strip_icc()/GettyImages-128113946-56a134ac3df78cf7726860d5.jpg)













:max_bytes(150000):strip_icc()/endothermic-reaction-examples-608179_FINAL-5d1c7be66fdb48878ae5842f4a873da6.png)









0 Response to "42 hot and cold packs chemistry"
Post a Comment