42 what factors affect the solubility of a substance
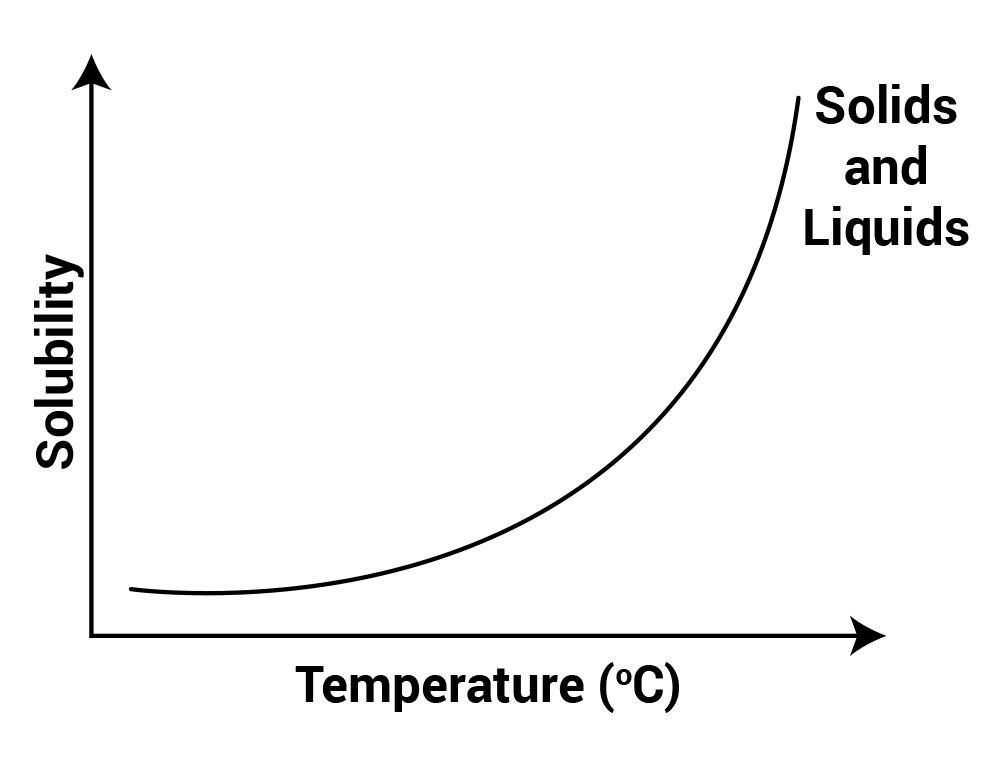
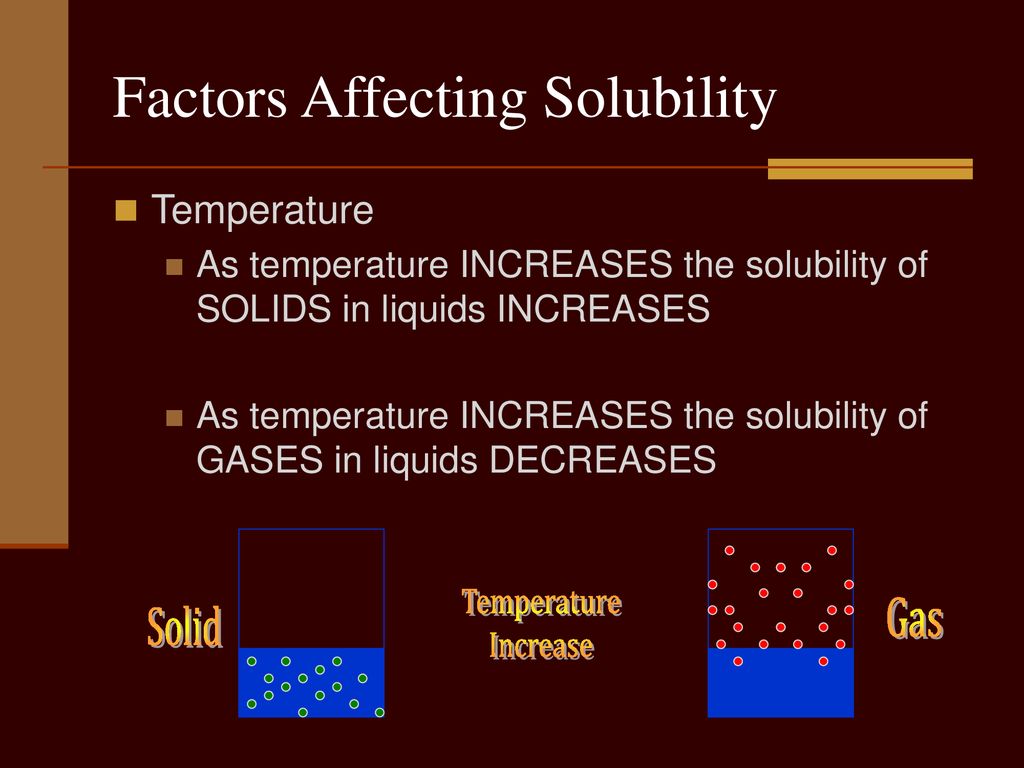
Factors affecting Solubility - GeeksforGeeks Factors affecting solubility are- Temperature- Solubility increases with temperature in the case of solid and liquid while in the case of gases, it decreases with an increase in temperature. Forces- It varies according to the type of intermolecular forces and bonds. Thus, like dissolves like and dissolving of unlike substances is challenging. What are the five factors that affect solubility? | - Soccer Agency The "10 factors affecting solubility" is a question that has been asked before. The five factors are temperature, pressure, concentration, surface area and the type of solvent. Solubility-related factors Temperature. Solubility rises as the temperature rises. Polarity.
What are the factors affecting the solubility? - toppr.com Verified by Toppr Solubility is the maximum amount of a substance that will dissolve in a given amount of solvent at a specific temperature. There are two direct factors that affect solubility: temperature and pressure. Temperature affects the solubility of both solids and gases, but pressure only affects the solubility of gases

What factors affect the solubility of a substance
Factors Affecting Solubility | Henry's Law and Rate of Dissolving - BYJUS Factors Affecting Solubility: The solubility of a substance depends on the physical and chemical properties of that substance. In addition to this, there are few conditions which can manipulate it. Temperature, pressure and the type of bond and forces between the particles are few among them. Temperature: Factors Affecting Solubility | Actforlibraries.org Factors that affect the degree of solubility are many and include temperature, pressure, nature of the solute and solvent (polarity), and rate of dissolution, which will now be discussed with examples. Common salt (solid NaCl) dissolves in water (solvent) to the extent of 36.0 grams in 100 grams (100 mls) of water at 20 degrees Celsius. Factors Influencing the Solubility of Drugs | Pharmlabs Identify factors that affect drug solubility. Explain the effects of temperature, multiple solutes, pH, and solute/solvent polarities (dielectric constant) on drug solubility. Describe the fundamental relationship between product formulation, drug solubility, and drug absorption, bioavailability, and therapeutic efficacy.
What factors affect the solubility of a substance. Chemical compound - Wikipedia A molecule is an electrically neutral group of two or more atoms held together by chemical bonds. A molecule may be homonuclear, that is, it consists of atoms of one chemical element, as with two atoms in the oxygen molecule (O 2); or it may be heteronuclear, a chemical compound composed of more than one element, as with water (two hydrogen atoms and one oxygen atom; H 2 O). Solubility: Definition, Formula, Examples, Notes - Embibe Factors affecting the solubility of a solid in a liquid. The important factors on which the solubility of a solid in a liquid depends are: ... (typically a liquid) and create a solution is known as solubility. A substance's solubility is mostly determined by the solvent employed, as well as temperature and pressure. The saturated solution ... Factors Affecting Solubility - Chemistry | Socratic Usually, increasing the temperature increases the solubility of solids and liquids. Increasing the temperature always decreases the solubility of gases. Explanation: When you add a solute to a solvent, the kinetic energy of the solvent molecules overcomes the attractive forces among solute particles. 4 Factors Affecting Solubility of Drugs - Ascendia Pharma 4 Factors Affecting Solubility pH Levels — pH measures the amount of hydrogen content in a solution—the more hydrogen ions, the lower the pH and vice versa. Solutions with strong pH levels fully dissociate and those with weak pH levels only partially dissociate. The pKa value is one method used to measure the strength of an acid.
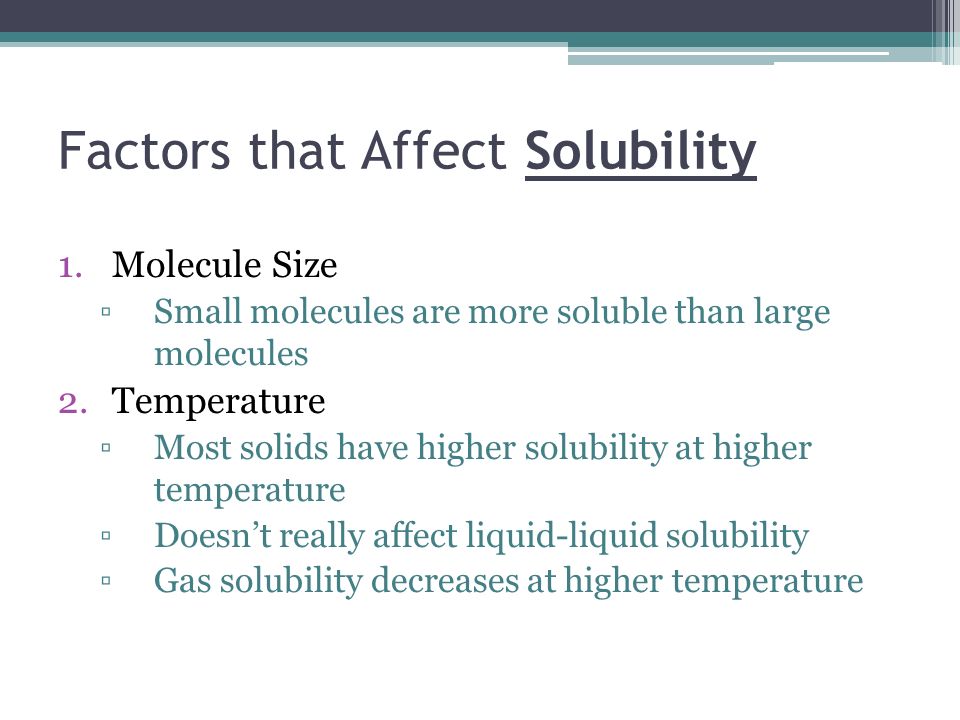
Urine - Wikipedia Urine is a liquid by-product of metabolism in humans and in many other animals. Urine flows from the kidneys through the ureters to the urinary bladder. Urination results in urine being excreted from the body through the urethra. Factors affecting solubility. What does solubility depend on? Factors affecting solubility ADVERTISEMENT Temperature Basically, solubility increases with temperature. It is the case for most of the solvents. The situation is though different for gases. With increase of the temperature they became less soluble in each other and in water, but more soluble in organic solvents. Polarity Factors affecting Solubility (solutions, examples, videos) Three Factors Affecting Solubility Molecule Size Pressure Temperature Show Step-by-step Solutions Temperature and Solubility of Solids Increased temperature usually increases the solubility of solids in liquids. According to the Second Law of Thermodynamics, increased temperature means a greater average velocity for the particles. The 6 Factors Affecting Solubility Main | Life Persona The 6 Factors Affecting Solubility Main. Main Factors affecting solubility Are the polarity, the effect of the common ion, the temperature, the pressure, the nature of the solute and the mechanical factors. Solubility is the ability of a solid, liquid or gaseous chemical (called the solute) to dissolve in solvent (usually a liquid) and form a ...
Factors Affecting Solubility - Solutions - PSIBERG The common factors that affect the rate of solubility are temperature, pressure, surface area, common ion effect, and, agitation (stirring). Below is the conventional criterion to understand how much soluble a solute is in a given solvent: Less than 1g/100ml, Insoluble. 1-3 grams per 100 ml of solvent, sparingly soluble. Factors That Affect Solubility - Highland there are four explanations why the solubility of a compound can differ from the solubility indicated by the concentrations of ions: (1) ion pair formation, in which an anion and a cation are in intimate contact in solution and not separated by solvent, (2) the incomplete dissociation of molecular solutes, (3) the formation of complex ions, and … Factors Affecting Solubility - Techiescientist Solubility is the maximum amount of solute that can be dissolved in one liter of solvent at a given temperature and pressure. The factors that affect solubility are temperature, pressure, and the nature of solute and solvent. However, the extent of the effect may vary from one solution to another solution. Solubility: Definition and Factors Affecting - Collegedunia The term solubility is a property of a substance that can be defined as the maximum of solute that will be dissolved in a known quantity of a solvent at a specific temperature. A solute is a substance which is dissolved in a solvent and can be solid, liquid or gas. ... Factors that affect solubility include - temperature, forces and bonds and ...
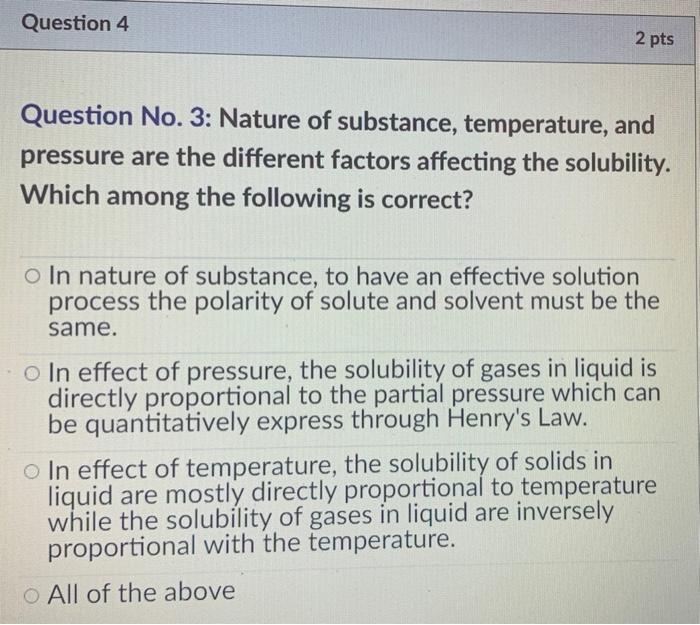
Factors Affecting Solubility: Explaination and Sample Questions Temperature and pressure are the two main factors that affect the solubility of a substance. Henry's law states that there is a direct proportion between a gas's solubility in a liquid solvent to the partial pressure (i.e., the pressure applied to the gas) above the liquid solution's surface. Read more: Mass Spectrometry
Factors Affecting Solubility | Boundless Chemistry - Course Hero solubility: The amount of a substance that will dissolve in a given amount of a solvent to give a saturated solution under specified conditions. Several factors affect the solubility of gases: one of these factors is temperature. In general, solubility of a gas in water will decrease with increasing temperature: colder water will be able to ...
Bioavailability - Wikipedia Other factors may include, but are not limited to: Physical properties of the drug (hydrophobicity, pKa, solubility) The drug formulation (immediate release, excipients used, manufacturing methods, modified release – delayed release, extended release, sustained release, etc.) Whether the formulation is administered in a fed or fasted state
What is Solubility? | The Chemistry Blog - ReAgent Chemicals What Factors Affect Solubility? Several factors affect the solubility of a solute. Primarily, the type of solvent is the main factor. Polarity of the solvent or solute: For example, gasoline is a nonpolar solvent, meaning it cannot dissolve polar substances like table salt (sodium chloride). However, it can easily dissolve nonpolar substances such as styrofoams.
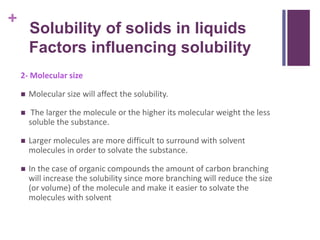
Factors Influencing Solubility - Pharmacy Scope Factors Influencing Solubility pH: Buffers: Cosolvency: Dielectric Constant: Solubilization: Complexation: Hydrotrophy: Chemical Modification of the Drug: Temperature: Salting-out: Particle Size: Molecular Size of Solvent Molecules: Molecular Shape of Solute Molecules: Macromolecules: Determination of Solubility
Solubility Equilibria: Effect of Common Ion and pH of the Solution ... The solubility equilibrium of calcium hydroxide would then shift towards the products, leading to an increased solubility in an acidic solution. 16.12: Factors Affecting Solubility Compared with pure water, the solubility of an ionic compound is less in aqueous solutions containing a common ion (one also produced by dissolution of the ionic ...
PDF Factors That Affect Solubility - Miss Braud's Science Site Factors That Affect Solubility One definition for an acid is a substance that lowers the pH of a solution as the acid's concentration is increased. A base, on the other hand, is a substance that increases the pH of a solution as the base's concentration is increased. But different acids have different effects on pH.
What factors affect the solubility of a particular substance? Answer: The overall solubility of a compound in any solvent is a sum of various intermolecular forces and intramolecular forces. If the solvent/solute interactions (molecules or ions) is a greater force than the solute/solute forces (molecular or ion/ion interactions) there is a good chance that ...
Solubility - Wikipedia Solubility is often said to be one of the "characteristic properties of a substance", which means that solubility is commonly used to describe the substance, to indicate a substance's polarity, to help to distinguish it from other substances, and as a guide to applications of the substance.
Solubility - Definition, Types, Factors Affecting, Examples Factors affecting the solubility of a liquid in liquid: Pressure: Pressure has a significantly greater impact on gases than it does on solids and liquids. When a gas's partial pressure rises, so does the likelihood of its solubility. CO 2 is bottled under high pressure in a soda bottle, for example.
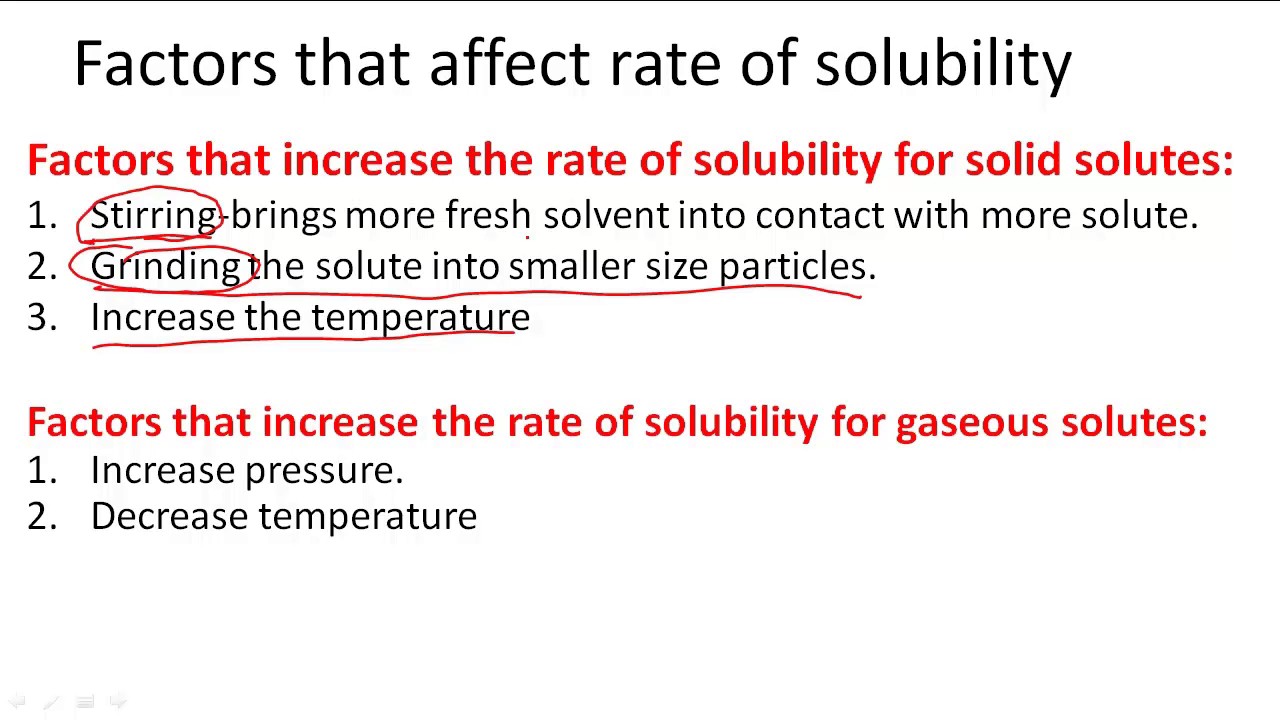
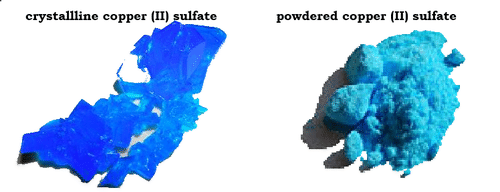
Formation of the solution & factors affecting the solubility process ... Shaking has the same effect of stirring process , The necessary factors to decrease the solubility time ( increase the solubility speed ) are : Heating . Stirring . Increasing the amount of solvent . Decreasing the amount of the solute . Grinding the solid materials . Types of mixtures in terms of homogeneity
Factors Affecting Solubility - Henry’s Law - VEDANTU Factors Affecting Solubility of Substances . Here, the solvent is taken as a liquid, and the solute will be taken for all three states to help you understand these factors that affect solubility. Liquid as solvent. Solids as solvent. Gases as solvent. Read further to learn in detail about the 3 factors that affect solubility. Liquid as Solvent
What is Solubility? - Definition, Solubility Product ... - BYJUS Let us first define solubility. For any substance, solubility is the maximum amount of solute that can be dissolved in a given solvent at a particular temperature. Now our concern is gas solubility in liquids. The gas solubility in liquids is greatly affected by temperature and pressure as well as the nature of the solute and the solvent.
DOC Solubility and Factors That Affect Solubility An example of this is Soda. The solubility of the carbon dioxide gas decreases when a soda is warm, making the soda flat. Pressure -- For solid and liquid solutes, changes in pressure have practically no effect on solubility. For gaseous solutes, an increase in pressure increases solubility and a decrease in pressure decreases solubility.
Factors Influencing the Solubility of Drugs | Pharmlabs Identify factors that affect drug solubility. Explain the effects of temperature, multiple solutes, pH, and solute/solvent polarities (dielectric constant) on drug solubility. Describe the fundamental relationship between product formulation, drug solubility, and drug absorption, bioavailability, and therapeutic efficacy.
Factors Affecting Solubility | Actforlibraries.org Factors that affect the degree of solubility are many and include temperature, pressure, nature of the solute and solvent (polarity), and rate of dissolution, which will now be discussed with examples. Common salt (solid NaCl) dissolves in water (solvent) to the extent of 36.0 grams in 100 grams (100 mls) of water at 20 degrees Celsius.
Factors Affecting Solubility | Henry's Law and Rate of Dissolving - BYJUS Factors Affecting Solubility: The solubility of a substance depends on the physical and chemical properties of that substance. In addition to this, there are few conditions which can manipulate it. Temperature, pressure and the type of bond and forces between the particles are few among them. Temperature:



























0 Response to "42 what factors affect the solubility of a substance"
Post a Comment