45 how does soap affect the surface tension of water
8 BEST Water Softener Shower Heads in 2022 - Kitchen Infinity 23/09/2022 · Soft water is also incredibly beneficial for your skin and hair and enhances the natural shine. A shower water softener does just this! The harmful mineral ions in normal, hard water keep it from dissolving completely with your body wash, and instead, it forms a nasty layer of soap scum. With a soft filtered shower head, you can easily prevent ... Electrolysis of Water Experiment | Science project - Education Magic Soap Experiment. Activity. Magic Soap Experiment. Your child will do a fun science experiment using soap and pepper flakes to help understand the concepts of water molecules and the surface tension of water. 2nd grade . Science . Activity. Water Purification Experiment: Removing Chlorine From Water. Science project. Water Purification Experiment: Removing …
8 Best Dishwasher Detergents: Reviews (For Cleaning) In 2022 11/12/2020 · It contains chemicals - surfactants - that work to decrease the water surface tension. This means that the water does not settle in droplets on your dishes, but instead it rolls right off without settling and leaving those unsatisfactory spots. Many detergent tablets and pods come with Rinse Aid within the mixture so that you don't have to add it separately.
How does soap affect the surface tension of water
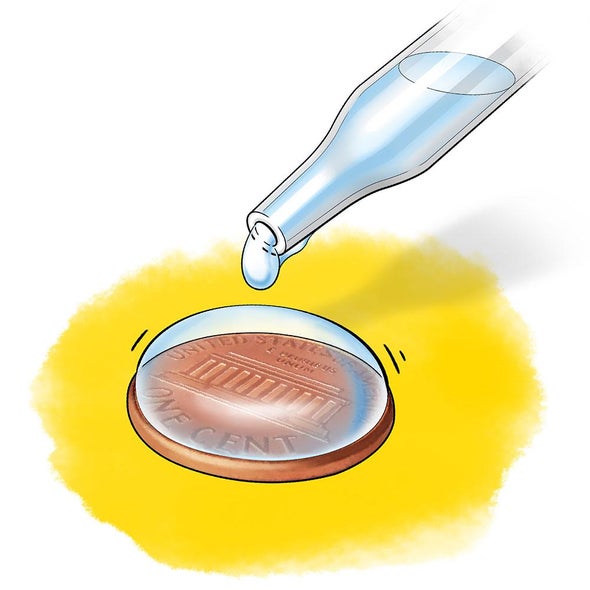
How is the Surface Tension of Water Affected by Soap? - The Biology Corner Procedure: Surface tension can be measured and observed by dropping water (drop by drop) onto a penny. Experiment : Carefully use a dropper to place drops of water on a penny. Count how many drops of tap water can fit on your penny. Do four trials and take an average. Repeat the process using water that has soap in it. PDF How is the Surface Tension of Water Affected by Soap? - Biology Question: How does soap affect the water's surface tension? 2. Develop a hypothesis that answers the experimental question. Write your hypothesis below. 3. Test your hypothesis by comparing the number of drops of tap water that can fit on a penny to the number of drops of soapy water that can fit on a penny. Because water drops may vary ... Detergents And Surface Tension - Action of Detergents | BYJU'S This is where detergent or soap comes in. Adding detergent to water reduces its surface tension. Detergents and soaps have something called surface-active agents, or surfactants for short. These surfactants reduce the surface tension value of water and enhance its ability to stick to things. Water sticks to the clothes and, because of this, can ...
How does soap affect the surface tension of water. Lab: How is the Surface Tension of Water Affected By Soap? Surface tension can be measured and observed by dropping water (drop by drop) onto a penny. The number of water drops that can fit on a penny will surprise you. Initial Observation: , Observe surface tension by seeing how many drops of water can fit on a penny. Number of Drops ___________, 2. Soapy Surface Tension | Science project | Education.com Liquid soap, Experimental Procedure: Fill a plastic cup with water. Dip the dropper into the cup, and suction in some water. Place drops of water from the dropper onto a penny, counting how many it takes for the water to spill off the penny. This is the number of drops it takes until the surface tension breaks. Dry off the penny. When Does Blood Pressure Medicine Start To Work When Does Blood Pressure Medicine Start To Work. Posted by High Mountain Institute; Date ... Does soap affect surface tension of water - YouTube 17 subscribers, This is a video of my students testing the surface tension of water. The first group uses tap water, and the second uses soapy water. Even though it is not great quality, you should...
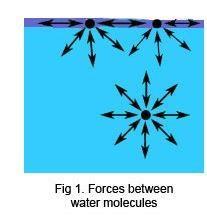
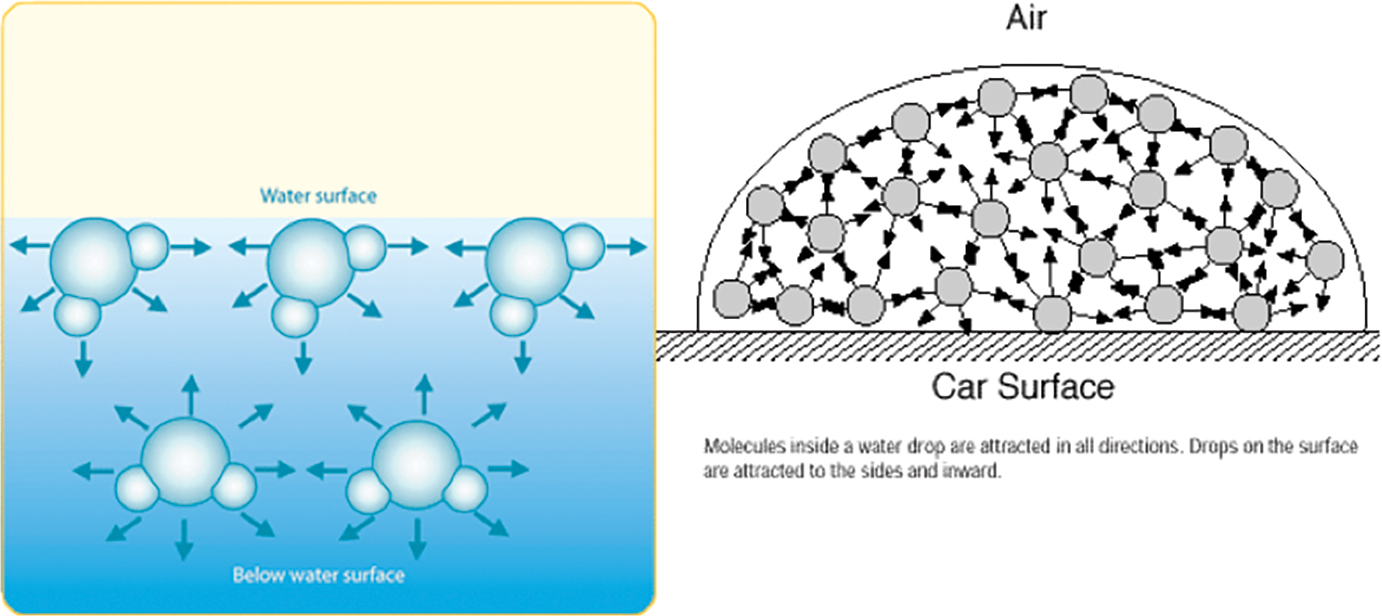
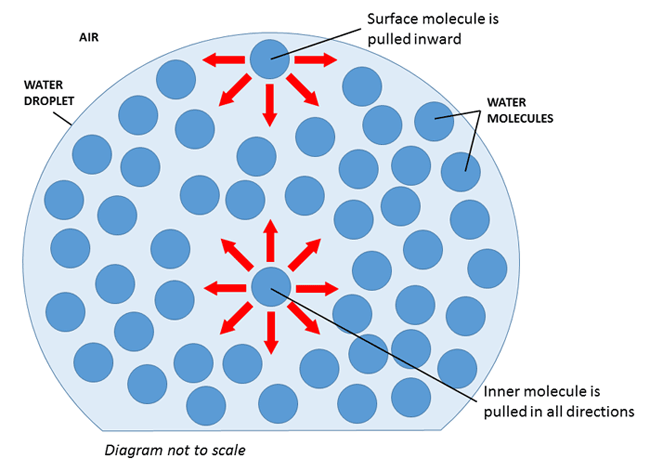
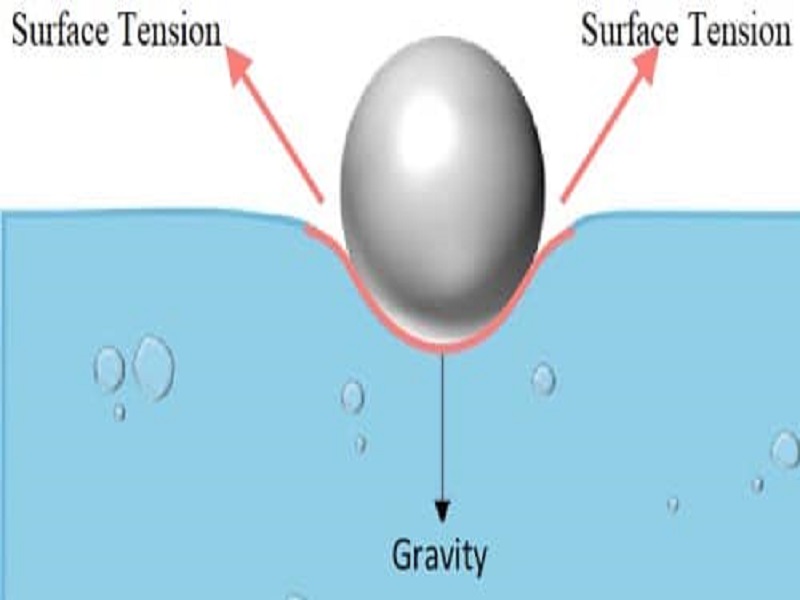
How will soap affect the surface tension of water? - Answers What effect does soap have on surface tension of water? Soap will lower the surface tension of water. Like any surfactant soap will lower the surface energy by disrupting the strong inter-molecular... Surface Tension and Water | U.S. Geological Survey Due to the surface tension, small objects will "float" on the surface of a fluid, as long as the object cannot break through and separate the top layer of water molecules. When an object is on the surface of the fluid, the surface under tension will behave like an elastic membrane. Examples of surface tension, Does Soap Affect the Surface Tension of Water - StudyMode I think that the surface tension of soapy water will be less than that of freshwater because H, 2, 0 has strong polar bonds, and when water is mixed with soap the polar bonds which help to bind the water together are weakened, thus lessening the surfacetension. Materials: Has water a surface tension? Explained by FAQ Blog What surface tension affects water? As explained, the cohesive force between the molecules causes surface tension. The stronger the cohesive force, the stronger the surface tension. ... Some liquids such as oil and kerosene can destroy surface tension in water. Adding soap or detergent reduces surface tension in water. Increasing the ...
How Does Soap Affect The Surface Tension Of Water - Phdessay Calculations Tap Water - (37+32+44)/ 3= 37 Soapy Water- (16+14+10)/3=13 -Conclusion In this experiment we confirmed the hypothesis that soap affects water by lowering surface tension thus lowering the amount that can fit on a penny. Post Lab, Explain what surface tension is/ Surface tension is water's ability to stick to itself. Detergents and Surface Tension - Explanation, Effect ... - VEDANTU Soap molecules contain long chains of hydrogen as well as carbon atoms that differentiate the water molecules from each other by decreasing their surface tension. As a result, there is a rise in the distance between water molecules. If there is no surface tension, the water molecules will evaporate immediately. 2-4-D Amine Mix Ratio Per Gallon | What Weeds Does It Kill If a ratio of 2.5oz of 2,4-D per gallon of water is used, across 400 sq ft, there is no risk. Using incorrect mix ratios can lead to scorching or burning of the surrounding vegetation. Also, excessive use of diluted 2,4-D can also lead to surface runoff, into nearby waterways, creating a potential risk to vulnerable wildlife. How To Apply 2,4-D ... Surface Tension: Causes, Examples and Dimensions - Collegedunia 02/09/2022 · Surface Tension is the tendency of a fluid to shrink into a minimum surface area. It is based on the principle that the molecules of the liquid at the surface area are in a different state than those in the center of the liquid. Surface tension allows objects with a higher density than water such as insects and razor blades to float on the water surface.
Water Properties | U.S. Geological Survey 22/10/2019 · Learn how "soft water" and "hard water" can affect how soap works. Learn More. link . June 6, 2019 Surface Tension and Water Surface tension in water might be good at performing tricks, such as being able to float a paper clip on its surface, but surface tension performs many more duties that are vitally important to the environment and people. Find out all about surface …
Does soap affect water's surface tension? by Zach Merrill - Prezi 3. Now put a drop of dish soap in the water. This will bind with the water molecules, interfering with the surface tension. 1. Start with a cup of water and some paperclips. 2.Gently lay a paperclip flat on the surface of the water. (This is tricky — it may help to place a piece of paper towel slightly bigger than the paperclip in the water.)
How Dish Soap Works - Water Surface Tension Experiment As explained above and in our demonstration, soap also lowers the water surface tension, this means rather then the water molecules sticking together tightly because of their strong attraction to one another, the water is now able to mix with the grease better, and thus help to clean off the grime. Add Tip, Ask Question, Comment, Download,
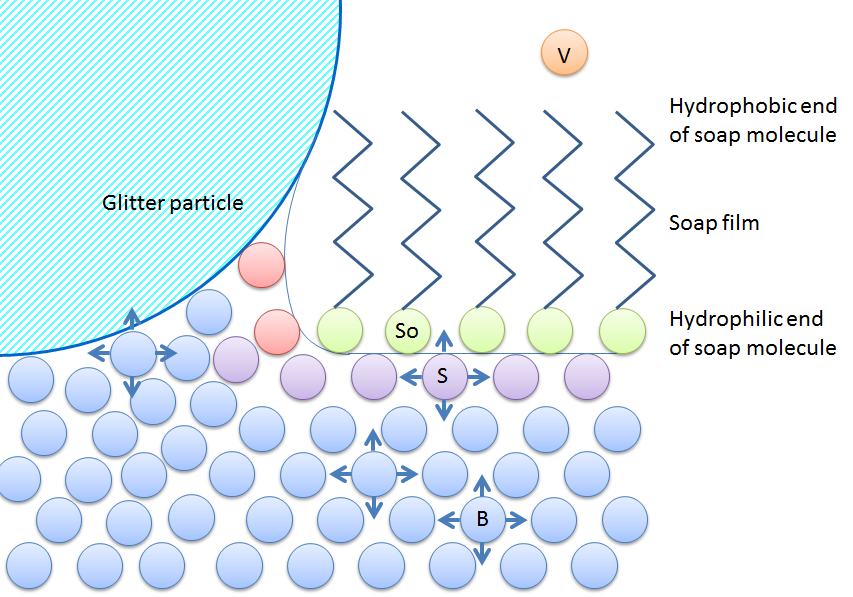
Does Soap Affect the Surface Tension Water? by Grace Allman - Prezi When soap is added to water, it clumps to at the surface because its molecules have two ends, one end repels water while the other end is attracted to water. It weakens the bonds between the water molecules. Hypothesis, Discussion, My results supported my hypothesis, pure water has a stronger surface tension than water with soap.
5 Experiments of Physical and Chemical Changes - Utah … A physical change as salt is dissolved in water does not affect mass. Find the mass of the small cup filled with 20 ml of water and the container that holds the salt (all at the same time). Predict whether the mass will change after the salt is mixed into the water. Add the salt to the water and mix the solution with a spoon. Be sure to not lose any of the salt or water during the mixing. …
Biology 1: How Is Surface Tension of Water Affected By Soap? - Quizlet The independent variable was the soapy water. Identify the dependent variable in the experiment. The dependent variable was the amount of drops of each type of water that it took to cover the surface of a penny. What if the experimental question was "How does sugar affect the surface tension of water?" Describe how you would answer this ...
Comprehensive review of the interfacial behavior of water/oil ... However, water flooding into an oil reservoir has a limited capacity to produce the remaining oil in the reservoir due to the interfacial tension (IFT) between oil and injected water. To address this issue, different types of chemicals, e.g., surfactants, alkaline, and nanoparticles, can be employed to reduce the IFT between oil and injected water to enhance the water flooding performance [ …
Soap and the effect it has on the Surface Tension of Water Soap is a natural surfactant, which is short for "surface active agent." Surfactants reduce the surface tension of water. Water will normally hold to itself because each water molecule is...
How Shampoo Works and the Chemistry Behind It - ThoughtCo 08/09/2019 · Here is a look at shampoo chemistry, including how shampoos work and why it's better to use shampoo than soap on your hair. What Shampoo Does . Unless you've been rolling around in mud, you probably don't have hair that is truly dirty. However, it may feel greasy and look dull. Your skin produces sebum, a greasy substance, to coat and protect hair and the hair …
Does Soap Affect The Surface Tension Of Water [pd49oxk08o49] Question: How does soap affect the water's surface tension? Hypothesis: I think that the surface tension of soapy water will be less than that of fresh water because H20 has strong polar bonds, and when water is mixed with soap the polar bonds which help to bind the water together are weakened, thus lessening the surface tension. Materials ...
PDF How is the Surface Tension of Water Affected By Soap? Soap? Introduction: Surface tension refers to water's ability to "stick to itself". Surface tension can be measured and observed by dropping water (drop by drop) onto a penny. The number of water drops that can fit on a penny will surprise you. 1. Initial Observation: Observe surface tension by seeing how many drops of water can fit on a penny.
Soap's Effect On The Surface Tension Of Water | ipl.org Soap disrupts the hydrogen bonds in water, which are the building blocks that keep water molecules together, so when soap severs those bonds, soap causes the water to overflow and the surface tension breaks. By adding more soapy water to the penny, the soapy water's surface tension will break faster than the tap water.
Why does soap reduce the surface tension of water? - Answers the surface tension has bonds, and the soap breaks those bonds, so if the soap water is put onto a surface.. it will slip off What effect does soap have on surface tension of water? Soap will ...
How does soap affect water surface tension? - Quora The surface tension of water is high due to the dipole nature of water, meaning that the water molecules pull on each other by electrical charge. Soap and detergents interrupt this layer, reducing the surface tension. A visual, though not scientifically accurate, would be a set of magnetic marbles that form a sheet.
What is the way the surface tension of water is affected by the ... The surface tension of water is high due to the dipole nature of water, meaning that the water molecules pull on each other by electrical charge. Soap and detergents interrupt this layer, reducing the surface tension. A visual, though not scientifically accurate, would be a set of magnetic marbles that form a sheet.
Measure Surface Tension with a Penny - Scientific American Procedure. Fill the medicine dropper with water. Now carefully add one drop of water at a time to the top of the penny. Hold the medicine dropper just above the top of the penny (not touching it ...
How Does Soap Affect Water Surface Tension? - BehindTheWash Unlike the water molecules, the soap molecules do not stick together. One end attracts the water molecules while the other end repels them causing the solid objects on top of the water to move along with the scattered molecules. This polarity breaks the tension in the water's surface effectively.
Detergents, soaps and surface tension - RSC Education A diagram of a detergent or soap molecule, which is responsible for breaking down surface tension, When the drop of detergent is added to the powdered surface, the initial effect is to draw the powder back to the edges very rapidly as the detergent molecules form their own surface layer with a lower surface tension than the water.
Detergents And Surface Tension - Action of Detergents | BYJU'S This is where detergent or soap comes in. Adding detergent to water reduces its surface tension. Detergents and soaps have something called surface-active agents, or surfactants for short. These surfactants reduce the surface tension value of water and enhance its ability to stick to things. Water sticks to the clothes and, because of this, can ...
PDF How is the Surface Tension of Water Affected by Soap? - Biology Question: How does soap affect the water's surface tension? 2. Develop a hypothesis that answers the experimental question. Write your hypothesis below. 3. Test your hypothesis by comparing the number of drops of tap water that can fit on a penny to the number of drops of soapy water that can fit on a penny. Because water drops may vary ...
How is the Surface Tension of Water Affected by Soap? - The Biology Corner Procedure: Surface tension can be measured and observed by dropping water (drop by drop) onto a penny. Experiment : Carefully use a dropper to place drops of water on a penny. Count how many drops of tap water can fit on your penny. Do four trials and take an average. Repeat the process using water that has soap in it.

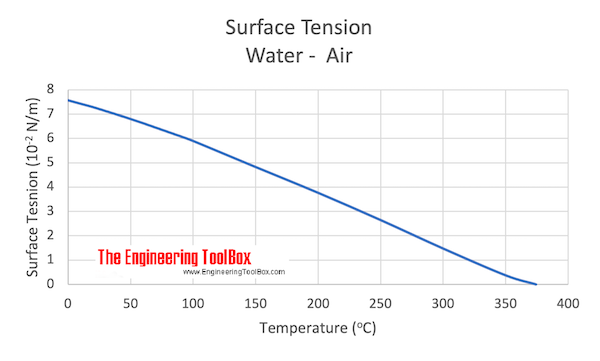







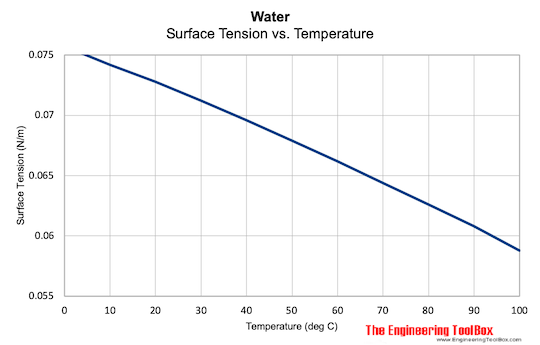








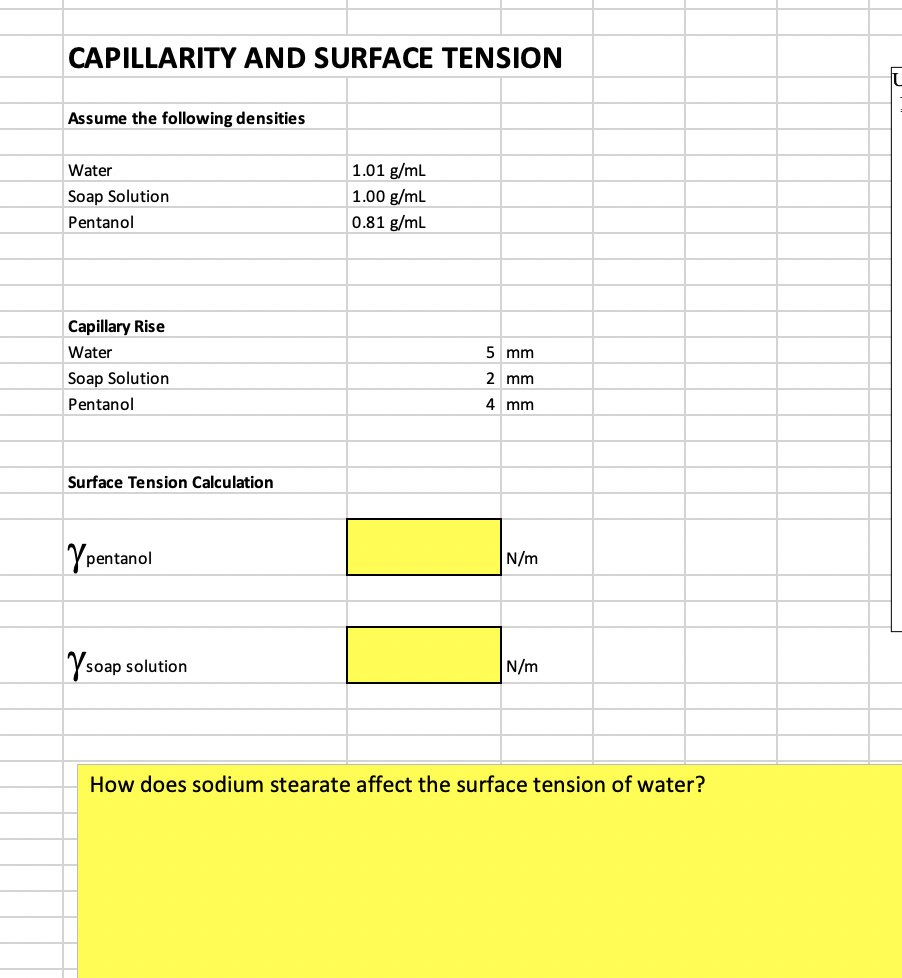
![Solved table3 Observations for Surface Tension 18 pt.] ADDED ...](https://media.cheggcdn.com/media%2F9ec%2F9ec7eb96-5be8-40d5-a13d-5fcf845bc062%2Fimage)

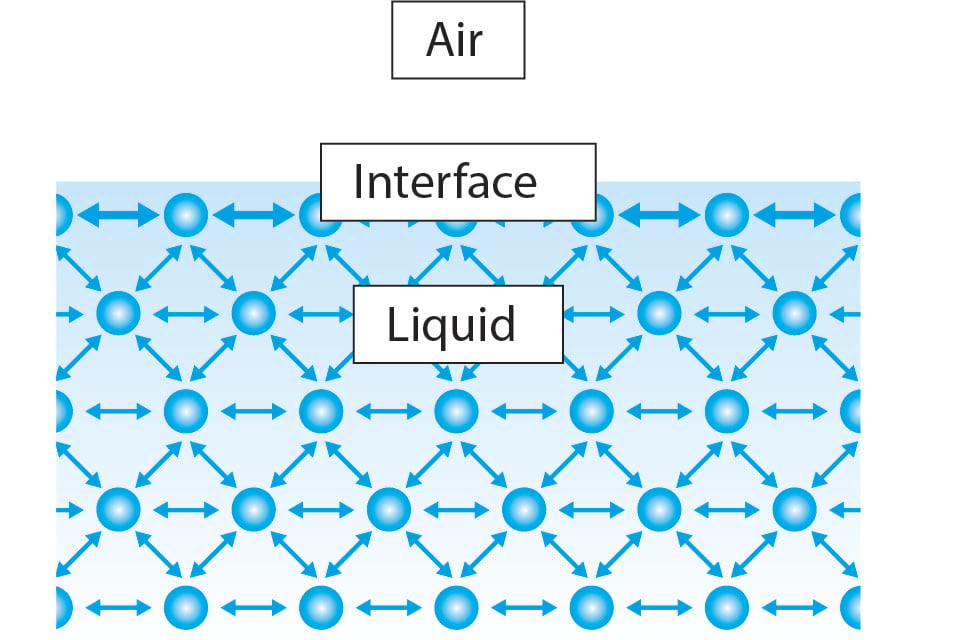









0 Response to "45 how does soap affect the surface tension of water"
Post a Comment