40 chemistry in a bag
PDF FUSE Chem in a bag - csep.cnsi.ucsb.edu 4. Have each group open a zip lock bag and add one teaspoon of any powder and another teaspoon of a different powder to their bag. (The powders will be in cups on a tray at each table) 5. Wait until each group has completed the above and instruct them to select a cup containing liquid (about ½ oz) from a tray that you bring around. PPT Chemistry in a Ziploc Bag: Mini-lesson The bag fills with carbon dioxide gas (see manual). The bag continues to feel cold because heat is being absorbed. Evidence for a Chemical Reaction Tell students what evidence to look for to determine if a chemical reaction occurs: a color change, a gas given off, temperature change, or the formation of a precipitate.
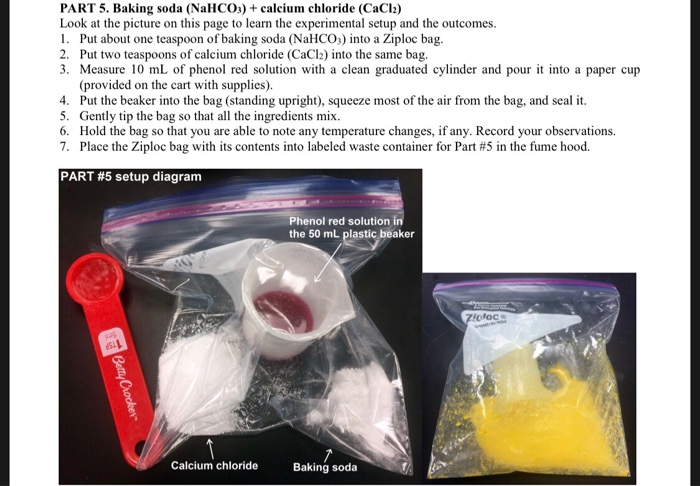
PDF Chemistry in a Ziploc Bag - chymist.com Put 2 spoonfuls of calcium chloride (CaC1 2) in the same bag. Record any observations. Measure 10 mL of the phenol red solution into the marked plastic vial. Place the vial UPRIGHT inside the Ziploc® bag, carefully squeeze most of the air out of the Ziploc ® bag, and seal it. Spill the phenol red solution out of vial by tilting the sealed ...

Chemistry in a bag
Classroom Resources | Chemistry in a Bag | AACT Measure 30 mL of distilled water and pour it into bag 1. Repeat for bags 2-4. Add 5 ml of the universal indicator to each bag. Note the color in your data table. Squeeze out the any excess air and seal the bags. Measure 1 gram (or use 3 nuggets) of Calcium, and add it to bag 1. Make sure the bag is tightly sealed. Chemistry in a Bag - The Art of Teaching Science Inquiry Procedure 1. Measure one spoonful of calcium chloride and place it into the ziplock bag. 2. Add one spoonful of sodium bicarbonate (baking soda) to the bag. Zip the bag closed and shake it to observe for any evidence of a chemical change. 3. Pour 10 mL of cabbage juice into a small vial. Chemistry in a Baggie | Central Coast Science Project ... This is a chemical change that produces no heat but does result in an acidic solution, so the cabbage juice turns pink. The acidic protons also react with the bicarbonae ions to make water and carbon dioxide gas, which inflates the bag. This chemical reaction also pulls in heat from the surroundings, so the bag becomes cold as well.
Chemistry in a bag. PDF 1 Chemistry in a Bag LAB - tatscience.weebly.com Chemistry in a Bag NAME: _____ In this lab you examine the variables in a chemical reaction and differentiate between a physical and chemical change. Materials: 3 Zip Top Bags 4 tsp. calcium chloride 4 tsp. Sodium Bicarbonate 3 Small Plastic Cups 10 mL Phenol Red 60 mL Water Scale Procedure: Part 1: 1. Add 1 tsp sodium bicarbonate to a zip top ... Kitchen Chemistry - A Sweet Treat in a Bag! — Pennsylvania 4-H Kitchen Chemistry - A Sweet Treat in a Bag! Kitchen Chemistry - A Sweet Treat in a Bag! Age Appropriateness: Elementary through High School. Suggested amount of time for lesson 20-30 min. Supplies Needed. Quart-sized zip-top bag (freezer bags work best and you could double bag your ingredients to keep from having to rinse the salt off ... PDF Chemistry in a Bag Lab - DIXIE MIDDLE SCHOOL SCIENCE Procedure Bag 4: 1. Place 10 mL of Potassium Iodide solution into the bag. 2. Using a dropper, add 3 drops of Lead Nitrate solution to the liquid in the bag one drop at a time. 3. Look, listen and feel. Record your observations: (color changes, gas being produced, heat being produced, precipitate, etc). Chemistry in a Bag--Analysis and Results PDF Lab-Chemistry in a Bag - DIXIE MIDDLE SCHOOL SCIENCE Procedure Bag 3: 1. Place 1 teaspoon of baking sodainto the bag. 2. Place ½ teaspoon of calcium chlorideinto the bag. 3. Mix the two powders togetherin the bag. 4. Add 5 mL of Phenol Redto the film canister. 5. Add 25 mL of water to the film canister. 6. VERY CAREFULLY- lower the film canister containing the 30 mL of liquid upright into the bag.
Baggie Chemistry Experiments - ThoughtCo An ordinary ziplock bag can unlock a world of interest in chemistry and in the reactions within and around us. In this project, safe materials are mixed to change colors and produce bubbles, heat, gas, and odor. Explore endothermic and exothermic chemical reactions and help students develop skills in observation, experimentation, and inference. Chemistry in a Bag - STEM-Works Students will learn all about the concepts of chemical reactions. Worksheets are provided along with the activity to assess what they observe, and what they learned from the experiment. Do this activity. PDF Chemistry In a Bag - Weebly Chemistry In a Bag Science & Matter Purpose: To make observations and inferences; and to design an experiment in order to isolate variables. Materials: · Your Lab Notebook · Scoopula · Goggles · Balance · Zip lock bag · Weigh paper · Sodium bicarbonate · 10 ml graduated cylinder Reaction in a Bag - The Wonder of Science Reaction in a Bag. Description: This video shows a chemical reaction of sodium bicarbonate (baking soda), calcium chloride (road salt), and an indicator phenol red. The chemicals react to form calcium carbonate, sodium chloride, and carbon dioxide gas. This changes the pH inside the bag resulting in a color change in the phenol red.
PDF Ziploc™ Bag Chemistry Countertop Chemistry from The Science House Teacher's Notes 1. a) There is a physical change in the first bag. See number 5 below for explanation b) A physical change occurs in the second bag. c) In the third and fourth bags a chemical change occurs. See note 4 for the equation. 2. Phenol red can be used to show the presence of an acidic solution. Reaction in a Bag - YouTube Hold a reaction in your hand, literally, as students observe and ask questions.This video is part of the Flinn Scientific Best Practices for Teaching Chemist... PDF Chemistry in a Ziploc Bag ( th Grade) - Vanderbilt University Hold the bag upright over the plate. 2. Open the bag while a VSVS member goes around and adds a teaspoon of calcium chloride. 3. Add the phenol red solution (including water) to the bag and seal the bag. 4. Gentlyshake the contents of the bag while holding the bag over the plate. 5. PDF Chemistry in a Ziploc Bag Lesson Plan - Vanderbilt University Chemistry in a Ziploc Bag 2018-2019 VINSE/VSVS Rural GOAL: To use the scientific method to explain observations made when calcium chloride, sodium bicarbonate, water, and phenol red are mixed. Fits TN State standards: LESSON OUTLINE I. Introduction
Chemistry in a Bag experiment and explanation - YouTube Chemistry in a Bag experiment and explanation - YouTube When three substances are mixed, a series of changes is observed. A great hands-on experiment for observation by changing variables. When...
PDF Chemistry in a Bag - Van Andel Institute for Education Chemistry in a Bag Grade Level/Content6-8/Physical Science Lesson SummaryIn this lesson, students will collect and use evidence to identify what chemicals are responsible for specific observations. They will also learn how matter can undergo both physical and chemical changes. Estimated Time2, 45-minute class periods
Chemistry in a Baggie | Central Coast Science Project ... This is a chemical change that produces no heat but does result in an acidic solution, so the cabbage juice turns pink. The acidic protons also react with the bicarbonae ions to make water and carbon dioxide gas, which inflates the bag. This chemical reaction also pulls in heat from the surroundings, so the bag becomes cold as well.
Chemistry in a Bag - The Art of Teaching Science Inquiry Procedure 1. Measure one spoonful of calcium chloride and place it into the ziplock bag. 2. Add one spoonful of sodium bicarbonate (baking soda) to the bag. Zip the bag closed and shake it to observe for any evidence of a chemical change. 3. Pour 10 mL of cabbage juice into a small vial.
Classroom Resources | Chemistry in a Bag | AACT Measure 30 mL of distilled water and pour it into bag 1. Repeat for bags 2-4. Add 5 ml of the universal indicator to each bag. Note the color in your data table. Squeeze out the any excess air and seal the bags. Measure 1 gram (or use 3 nuggets) of Calcium, and add it to bag 1. Make sure the bag is tightly sealed.
































0 Response to "40 chemistry in a bag"
Post a Comment